Contract Management In The Pharmaceutical Sector
Due to increased globalisation & widespread changes in the supply chain, compliance with regulations for the pharmaceutical sector is growing increasingly difficult. Often, these compliance burdens have a negative impact on an organization’s contracts, which are the foundation of all commercial interactions.
Contracts are essential in all transactions, whether they be buying from suppliers, conducting clinical studies, working with healthcare professionals or Contract Research Organizations (CROs) for clinical research, or interacting with wholesalers and Pharmacy Benefit Managers on the purchase.
As a guideline, a contract’s lifespan should be carried out in relevance to & accordance with the business. However, due to increasing complexities in the pharmaceutical sector and frayed lines of communication between various stakeholders, contracts are often delayed, misplaced, or not even completed.
These challenges are mainly due to the usage of manual or paper-based contracting systems, which sometimes result in businesses taking 5-10 days to get a single contract from draft to sign.
Smart contract management enables businesses to automate their contracts and adapt to the changing nature of compliance in the pharmaceutical sector.
What Is Contract Lifecycle Management?
Contract Lifecycle Management or CLM is the implementation of a series of actions aimed to aid the attainment of a contract’s goal, compliance with duties, and risk minimization or mitigation.
CLM involves a number of steps that account for every stage in a contract’s lifecycle –
- Contract Request: The legal or sales team places a request for a contract
- Contract Creation: Contracts are drafted instantly using custom templates
- Contract Negotiation: Stakeholders negotiate contracts online
- Contract Execution: eSignatures are used to remotely sign contracts
- Contract Storage: Contracts are tagged & stored in a central repository
- Contract Performance & Tracking: Contract intelligence tools track contract performance and create actionable insights
- Contract Renewal: Contracts are renewed after custom alerts are sent to notify stakeholders regarding contract expiry
- Commencement Date Management: The contract’s commencement date is clearly defined and tracked to ensure timely implementation of obligations.
Leveraging smart contract management and its automated processes not only reduces the expenses & man-hours involved in contracting but these smart systems can also help businesses create an audit trail for compliance and manage the risks associated with the pharmaceutical industry.
What is a Contract Research Organization?
A contract research organization (CRO) is a biomedical firm that provides research services to the pharmaceutical, biotechnology, and medical device sectors on a contract basis.
Some healthcare systems contract out traditional freedom, drug stockpiling acquisition, and transportation. A health framework may occasionally contract out medication administrations via retailer or non-revenue driven pharmaceutical outlets. Rethinking administrations can occasionally save expenses and increase profitability.
The decision to contract out or provide types of assistance in-house should be based on a careful examination of the impact on the entire store network, including costs, execution, the limit of the private area to provide the labour and products being referred to, and the limit of the wellness framework to screen the agreement.
When genuine rivalry occurs, the healthcare framework is equipped to drive the agreement, and sufficient resources are available to pay, reevaluating is likely to succeed.
Why are contracts essential in the pharmaceutical industry?
The pharmaceutical industry relies heavily on external vendors & suppliers, organizations for drug research & testing, hospital representatives, liaison officers with regulatory entities, and several stakeholders to ensure that operations run smoothly.
The relationship between a pharmaceutical company and any of these stakeholders is determined and performed according to contracts. Hence contract management is a crucial aspect for businesses in this sector. Here are a few ways that the pharmaceutical relies on contracts.
- Worker-for-hire security operations are used by drug organisations for a variety of reasons, the most common of which is because their offices are typically under construction. All of that is continuously changing, with new faces coming and leaving, but security should remain a constant and an activity’s major priority. Security contracts are the cornerstone of this relationship.
- According to industry estimates, the global market of clinical research organizations was worth $17.8 billion in 2007, and demand for their services is expected to expand at a 15% annual pace. With greater demands for research before piloting a drug, pharmaceutical companies will need to leverage smart contract management to ensure smooth operations.
- Pharma companies can use contract management to create policies that will protect them from force majeure situations and provide certain benefits, such as the ability to quickly issue clear, up-to-date risk management policies or the ability to deploy new policies and specifications to documents in bulk.
- Companies must complete a slew of applications to certify a new medication’s compliance with local Food and drug administration criteria before releasing it to the public. A lack of compliance increases the danger of delays and financial losses as a result of missed deadlines, not to mention the competition that can be avoided with efficient contract administration. Leveraging a smart contract management system can ease the compliance burden on businesses in the sector.
In a report, it was found that these research organizations now account for around half of the research workforce involved in drug and medical product development. They provide research sponsors with a comprehensive range of clinical technology solutions, such as study design consulting, investigator recruiting, study monitoring, and data analysis.
As per data, the market size of the clinical research organization between 2015-2024 (projected) can be figured from the following:
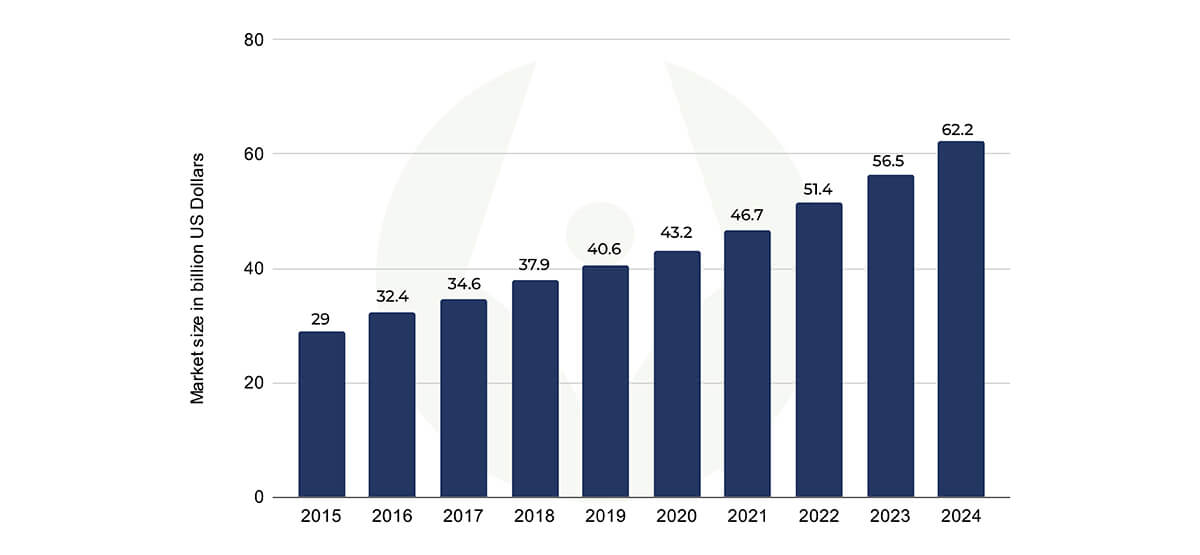
By 2023, it is expected that the market size of clinical CRO will reach some 57 billion U.S. dollars worldwide. This statistic shows the market size of clinical contract research organizations (CROs) globally from 2015 to 2024, in billions U.S. dollars.
Contract Types in the Pharmaceutical Industry
Smart contract management solutions offer custom templates for legal and sales teams at pharma companies to quickly draft boiler plate agreements in bulk. Here are a few of the most common agreements used by businesses in the pharmaceutical sector.
-
Agreement of Quality:
Constructing quality agreements is supported by parties involved in the cooperative manufacture of pharmaceuticals. They describe how each party would adhere to CGMPs. Supplier and retailer quality agreements state the terms relating to the type of materials or administrations provided to a manufacturing office supplying the medicine.
-
Agreement on Licensing:
The authorising firm provides an organisation in the host country the right to develop and market a drug under an authorising agreement. In exchange for its specific competence, the licensee compensates the authorised firm.
-
R&D Agreements:
Pharma organisations execute communitarian R&D cooperation agreements in which the collaborating parties agree to engage in creative work and the subsequent commercialization of a treatment.
The management of clinical preliminary studies is frequently subject to time constraints, with the element of time having a crucial role in the worldwide competition, particularly in the period preceding the start of a clinical preliminary study.
A pharmaceutical or biotechnology company’s success is dependent on its capacity to bring innovative medications to market, which puts increasing pressure on companies to move clinical trials through the pipeline faster and more cost-effectively.
Efficiency is key for contract research organisations (CROs), and time is literally money; yet, for all enterprises, this focus on speed must not come at the expense of patient safety or compelling effectiveness proof. Instead, businesses would do well to leverage automated contract solutions to expedite the contracting process and get medications to market quickly and cost-effectively without compromising on testing.
This is just one reason pharmaceutical companies need smart contract management; however, contract automation fulfills more than just this need.
Why Pharmaceutical Businesses Need Smart Contract Management :
- Contract management and process control are streamlined:
By simplifying, businesses can govern, organise, and automate contracts for any use case. Increase adoption by integrating Word, Outlook, and Salesforce and simplifying workflows throughout the organisation including legal, clinical, vendor management, marketing, and sales.
Overseeing each stage of the contract lifecycle process may take up contract managers’ time and energy, while also exposing them to the danger of human mistake when manually maintaining various documents.
Contract administrators may find it difficult to maximise contract performance throughout the contract lifecycle. Intelligent contract workflow automation lowers bottlenecks by connecting the relevant people at the right time with almost infinite job reminders and contract notifications.
-
Increase negotiation cycles and turnaround periods:
The last thing users want is a contract cycle that consumes too much of their time. With speedier internal and regulatory clearance channels, companies can get to market faster than rivals.
-
Plan better:
Gain insights into the financial and compliance performance to make better decisions. Transparency in income flows and royalties help the bottom line by allowing for better planning and budgeting of business’ intellectual property, grants, and other commercial agreements.
-
Improved CRO Relationships:
Customised applications have been developed for CRO interactions to complement the benefits of the ICM platform. The Clinical Trials Agreement and Budgeting app gives sponsors and contract research organisations (CROs) sophisticated capabilities for managing clinical trial agreements and tracking finances.
This enables organisations to assure compliance, increase coordination with CROs and locations, ensure agreement compliance; conveniently manage trial budget usage; and minimise contract cycle times by up to 80%.
-
Cross-channel Automation Reduces Risk:
Pharmaceutical companies today confront the challenge of increasing income, avoiding attrition, and retaining client relationships in an extremely competitive climate. This entails establishing a comprehensive strategy for fostering a strong process-centric & holistic approach to contracting, which is the primary goal of excellent contract lifecycle management (CLM).
Contract Management Trends in Pharmaceutical Industry
-
Outsourcing:
Any pharmaceutical business develops, manufactures, and markets its products using a combination of captive and outsourced resources, and outsourcing is definitely on the rise as corporations focus on core skills. According to the industry publication Contract Pharma, the market for outsourcing services is increasing faster than the pharmaceutical sector as a whole.
Now, a pharmaceutical company’s connection with its vendors is vital to its success, and these ties have evolved into much more collaborative ones.
Smart CLM enables pharma businesses to collaborate in real-time with vendors and suppliers, ensure uniformity in outsourced contracts and processes, and most importantly keep a strong check on their compliance activities.
-
Increasing Complexity In Supply Chains:
As firms outsource more, their supply chains get more sophisticated, as they must supervise work conducted across several countries to guarantee items are brought to market safely and on schedule. A single trial may involve numerous Contract Research Organizations (CROs), and it is frequently the duty of the sponsor to guarantee that all locations are compliant.
Smart CLM helps businesses organize contracts relating to CROs, track contract performance to ensure compliance, and handle increasing complexities using a smart CLM’s AI-powered contract insights.
-
Cost-cutting Measures:
Historically, the pharmaceutical sector has been driven by money. However, as reimbursement constraints and drug development costs have risen over the last decade, firms have placed a larger emphasis on cost containment.
The capacity of a corporation to optimise spending is hampered by manual contracting methods, which can be automated with ease using a contract management solution.
How Can Contract Life Management Help Your Business?
Enterprise contract management solutions, which include central contract repositories, automated workflows, and the tracking of obligations and costs, can assist pharmaceutical businesses in proactively addressing these difficulties.
Visibility into these complex supplier networks might be unattainable with a manual contracting approach. Back-to-back contracting circumstances, in which a vendor contracts with another vendor to fulfill an obligation, are especially challenging to track. Companies expose themselves to regulatory risks if they do not have complete insight into multi-tier supply networks.
To compete in today’s changing pharmaceutical sector, organisations must discover ways to speed their operations, defend against regulatory and financial risks, and improve procedures. Enterprise contract management can help with all these objectives.
Book a free demo with us now!
